What is Article 51 and why proposing a device to promote innovation?
Currently, the health system is encountering several difficulties related in particular to the emergence of new pathologies and the segmentation of healthcare funding. This situation forces the different parties to evolve within a constrained budgetary framework and often leaves the patient to coordinate his own care path.
Article 51 is an article of the French Social Security Financing Law of 2018 setting a new framework for launching experiments of a limited duration to address the previously mentioned issues.
This article offers field actors in the healthcare world the opportunity to test new approaches by deviating from the usual rules of organization and funding. More specifically, these innovations concern:
- The optimization of care channels and payment methods
- The reorganization of the patient pathway and the procedures for monitoring patients
- Changes in financial incentives for the prescription of certain drugs
What projects are eligible under Article 51?
To be eligible under Article 51, a project must:
- Belong to at least one of the categories of experiments provided by law, namely: innovative financing methods, innovative organization or relevance of health products.
- AND derogate from a rule of common law of the Social Security Code or the Social Action and Families Code or the Public Health Code.
- AND respect the selection criteria: the project must be feasible (operational), innovative (not previously implemented), reproducible (replicable in other territories) and efficient (favorable medico-economic impact).
However, Article 51 is not a request for a grant, a biomedical research project on human beings, an isolated financing of a technological tool/solution (without specifying the impact on the patient journey), a procedure for negotiating the price or reimbursement of a new product or service.
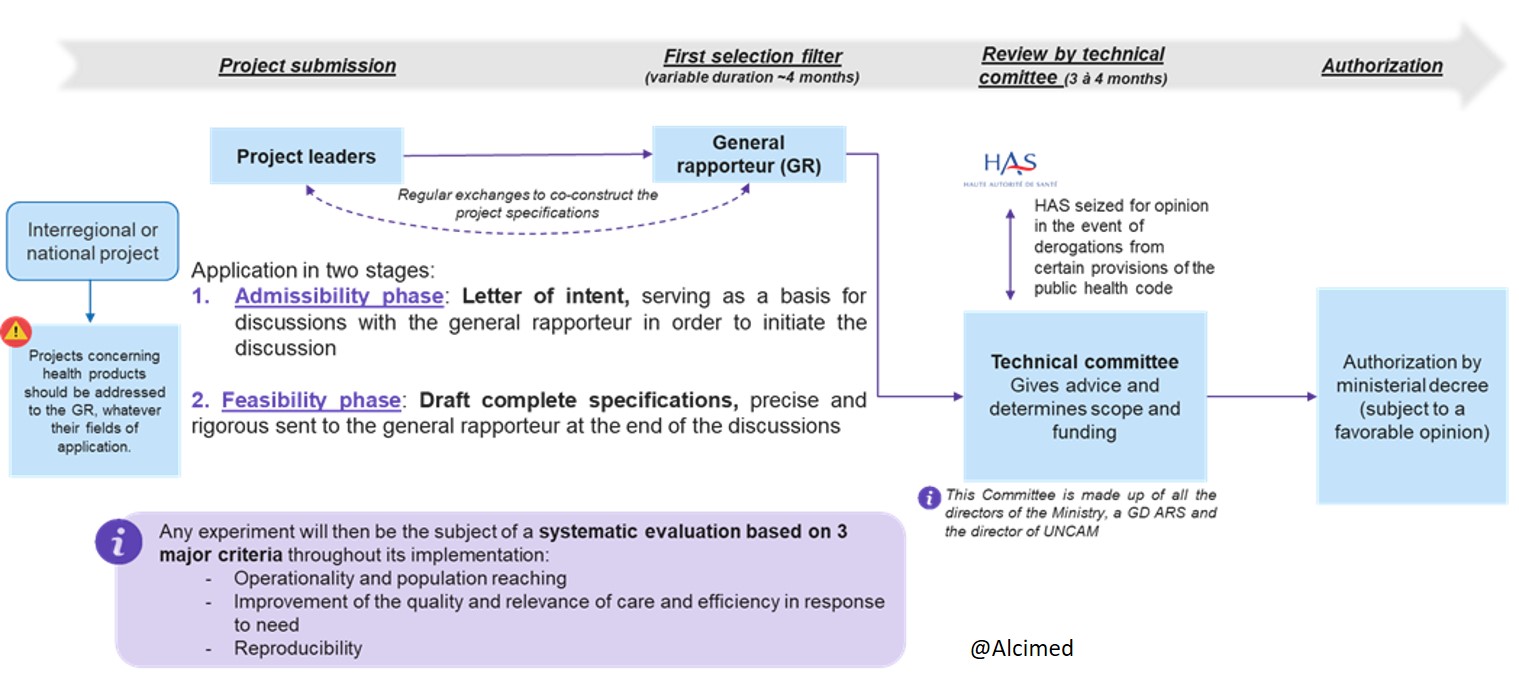
What is the procedure for submitting a project under Article 51?
The projects submitted go through an iterative and rigorous selection process, in four main stages:
- The submission of the project by the project holders
- The first selection filter by the General Rapporteur
- Examination by the technical committee made up of all the directors of the Ministry, a Director General of ARS (French Regional Health Agency) and the Director of the French National Union of Health Insurance Funds (UNCAM)
- Authorization by ministerial order or by order of a DG of ARS

In which framework are the experiments of Article 51 carried out?
Within the framework of this Article 51, there is no restriction on the nature of the project leader, it can be user associations, health establishments (public or private), federations, unions, health professionals, startups, home help professionals, complementary organizations or local authorities. In addition, this device is applicable to the public and private sectors, as well as to city medicine and hospitals, and is also applicable to the medico-social sector.
54 experiments initiated by stakeholders (outside ministries) are currently underway. These can be local, regional, interregional or national (although the latter option is not widely used). The duration of the experiment can vary from a few months to a maximum of 5 years.
In order to finance these experiments, the French Social Security Financing Law of 2018 created the fund for the innovation of the health system, the FISS. In addition, the regional intervention fund (FIR) can be mobilized to partly finance regional projects. As an indication, in 2019, the FISS had an endowment amounting to € 30M and the FIR to € 10M to finance the authorized experiments.
What happens once the experiment is authorized?
The innovation system of Article 51 is based on a “test & learn” approach. First, the experiments are tested. Then with the help of an evaluation throughout the experiment lessons are learned, and the model is adjusted accordingly. Levers for optimizing the health system are identified in order to generalize the experiment and integrate it into common law.
The evaluation is carried out throughout the implementation of the project and at the end of the experiment. It is co-managed by the CNAM (National Health Insurance Fund) and the DREES (Department of Research, Studies, Evaluation and Statistics) of the Ministry of Solidarity and Health. They rely on evaluation experts with different statuses (national institutions, researchers, etc.).
The evaluation is based on the construction of performance indicators, on qualitative approaches and on comparative methods to assess the impact of the experiment.
In short, the opportunities offered by Article 51 are multiple, carriers can offer new care pathways whether or not integrating new tools, new funding methods or promoting better use of health products. All this with the aim of improving the current health system and providing the best possible support to patients. Alcimed supports its clients in the construction of their project with a view to submitting their experimentation under Article 51 of the LSSF of 2018.
About the author
Pauline, Project Manager in Alcimed’s Life Sciences team in France