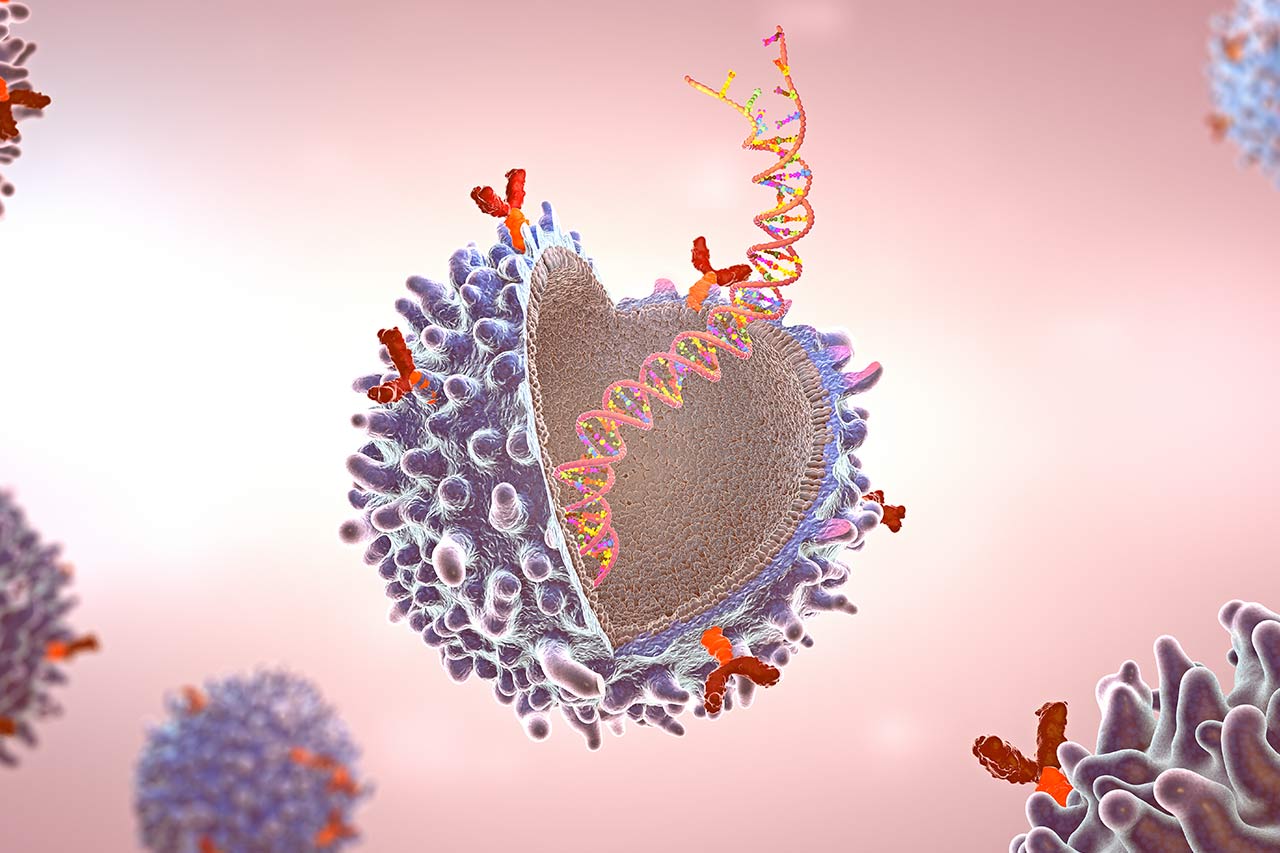
Chimeric Antigen Receptor (CAR) NK cell-based therapy has recently been drawing significant attention as a new therapeutic tool for cancer. NK cell refers to natural killer cells which are innate lymphocytes, allowing faster response to pathogens before adaptive immune system is being activated. Compared to T cell, NK cell is recognized as a better CAR base with stronger efficacy and safer clinical application due to its natural anti-tumor receptors and allogeneic compatibility. Moreover, CAR-NK cell therapy with its “off-the-shelf” potential will catalyze a new revolution in immunotherapy for cancer. In this article, Alcimed will shed lights upon these key privileges of CAR-NK cell therapy and identify its major challenges.
What is CAR-NK cell therapy?
CAR-NK cell therapy is a novel immunotherapy strategy by utilizing genetically-engineered NK cells to target specific cancer, which can be both liquid and solid tumor. NK cells can be initially acquired from various sources, including patients, healthy doners and laboratory (e.g. induced pluripotent stem cells—iPSC).
Genetic code of specific CAR receptors for target tumor will be incorporated into NK cell genome, and thereafter displayed on the cell surface. CAR-NK cells is enriched in favorable culture conditions before transfusion into the patients. Once in the blood circulation, CAR receptors will work as a “GPS navigator” to local NK cells by binding of target antigen protein on tumor. NK cells will subsequently release pore-forming proteins which cause cancer cells destruction.
Why could CAR-NK cell therapy go beyond CAR-T cell therapy?
NK cells are suggested as a superior CAR base due to its unique physiological characteristics. By replacing the base of CAR therapy with NK cells, it can remarkably resolve the problems of autologous restriction and severe adverse effects caused by CAR-T cell therapy.
NK cells “Off-the-shelf” potential
NK cells are not restricted by autologous setting, meaning that patients can receive allogenic NK cells from various sources without concerning about grafted-versus-host diseases (GVHD). This is because NK cells do not require human leukocytes antigen (HLA) matching as T cells do. This unique feature opens the gate to a streamlined manufacturing process of producing specific CAR-NK products in advance and increases the scalability without customization to individual patients. Moreover, NK cells acquired from iPSC are homogeneous population compared to ones from other sources, which ensures the standardization as “off-the-shelf” product.
CAR-NK cell therapy safer clinical applications
NK cells have shorter lifespan than other cytotoxic lymphocytes. This feature can dramatically mitigate the risk of on-target/off-tumor effect, which is a major issue in CAR-T cell therapy due to the memory T cells. Without staying in blood circulation for long term, left-over CAR-NK cells will less likely affect CAR-targeted healthy cells.
Furthermore, activated NK cells secrete a bounty of cytokines that are quite different from T cells. The latter tend to release various pro-inflammatory factors resulting in Cytokine Release Syndrome in CAR-T clinical applications. Excessive cytokines would circulate in the blood stream, which can cause damage to organs and even death. Fortunately, activated NK cells typically release IFN-gamma and GM-CSF which are not major contributors to the cytokine storm.
Stronger efficacy on targeting cancer cells
NK cells are naturally expressing a wide range of anti-tumor receptors. These receptors are found to be associated with the stress-induced ligands which are commonly identified on the surface of cancer cells. Rather than solely relying on the CAR target receptors, like CAR-T cell therapy, the reinforcement of binding these tumor ligands with the natural anti-tumor receptors makes CAR-NK cell therapy even more effective against the malignant cancer cells with vast heterogeneity.
What are the limitations for CAR-NK cell therapy development?
Although “off-the-shelf” and short-live are recognized as the superiorities of CAR-NK cell therapy, these privileges could also give rise to two challenges that impede the clinical practices.
Cryopreservation leads to weakened anti-tumor efficacy
To make the “off-the-shelf” product, cryopreservation is the common strategy for preserving the intact CAR-NK cells products under very low temperature. However, NK cells are very sensitive to freezing and thawing compared to other lymphocytes, which can negatively impact the killing efficacy after recovering from cold. GC cell (South Korea), one of the commercial leaders in CAR-NK cell therapy, has discovered a particular composition of freezing media which could improve the preservation of viability and function of NK cells. This advance freezing technology has dramatically accelerated the readiness of CAR-NK cell as “off-the-shelf” therapeutic products.
Short lifespan pruned the persistent therapeutic effect against invasive tumors
Due to short lifespan, CAR-NK cells will be gradually depleted after exercising the killing activities on cancers. Low persistence could lead to incomplete removal of cancer cells, especially for invasive tumors. In addition, NK cells do not produce memory cells that can persistently remain in the blood circulation. Therefore, tumor regression could occur, making the treatment ineffective. Researchers have been attempting different strategies to improve the in vivo persistence of CAR-NK cells, including multiple dosage of iPSC-derived NK cells, co-expressing cell survival triggers (sIL-15 or mbIL-15) on CAR construct and modifying CAR backbone structure to prevent in vitro over-activation.
Who are the key industrial players of CAR-NK cell therapy?
Nkarta Therapeutics has received $114 million series B investment on building up CAR-NK therapy research pipelines and manufacturing site with two of pipelines entering clinical trial phase. Kiadis Pharm has also developed multiple programs of CAR-NK therapy research focusing on both liquid and solid tumor treatments, one of which has been in phase II clinical trial.
CAR-NK cell therapy shows the potential to cause a future paradigm shift in cancer treatment. Better addressing the challenges of CAR-NK cell therapy would be the key determinant to become a leading player in future immunotherapy area. We, at Alcimed, will be continuously exploring the frontiers of knowledge in CAR-NK cell therapy and look into latest solutions for pushing forward the clinical applications.
About the author,
Xianjin, Consultant in Alcimed’s Healthcare team in Asia-Pacific